Gas Liquid And Solid Diagram
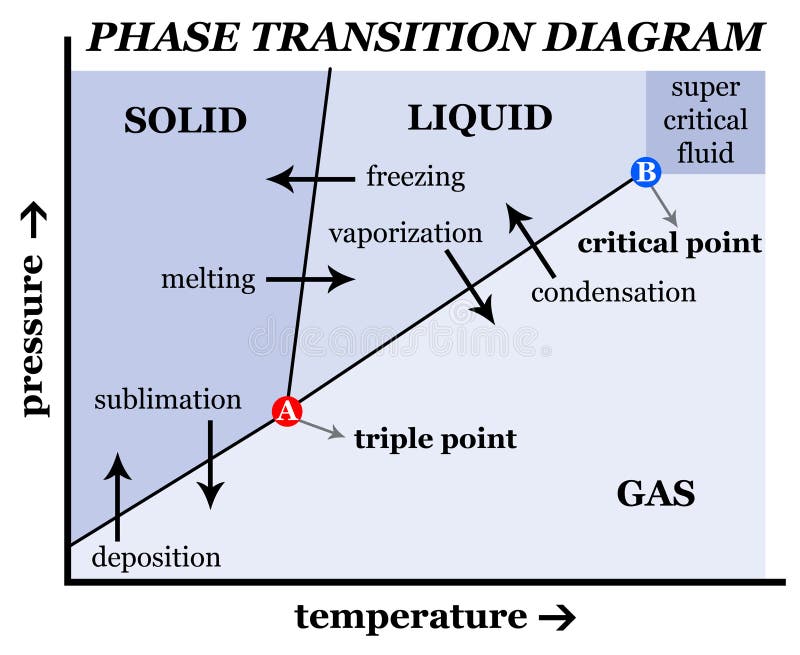
As we increase the temperature, the pressure of the water vapor increases, as described by the liquid-gas curve in the phase diagram for water ( Figure 10.31 ), and a two-phase equilibrium of liquid and gaseous phases remains. At a temperature of 374 °C, the vapor pressure has risen to 218 atm, and any further increase in temperature results.
Solids Liquids And Gases Images and Photos finder
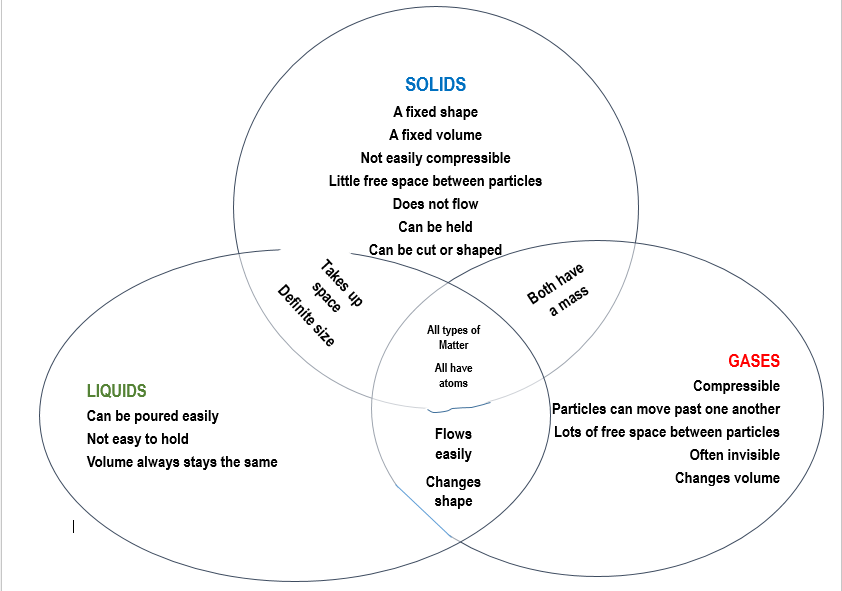
Key points Almost everything is made of particles. Particles can be atoms, molecules or ions. Particles behave differently in solids, liquids and gases. The particle model explains the.
Solid liquid gas stock illustration. Illustration of atom 83381916
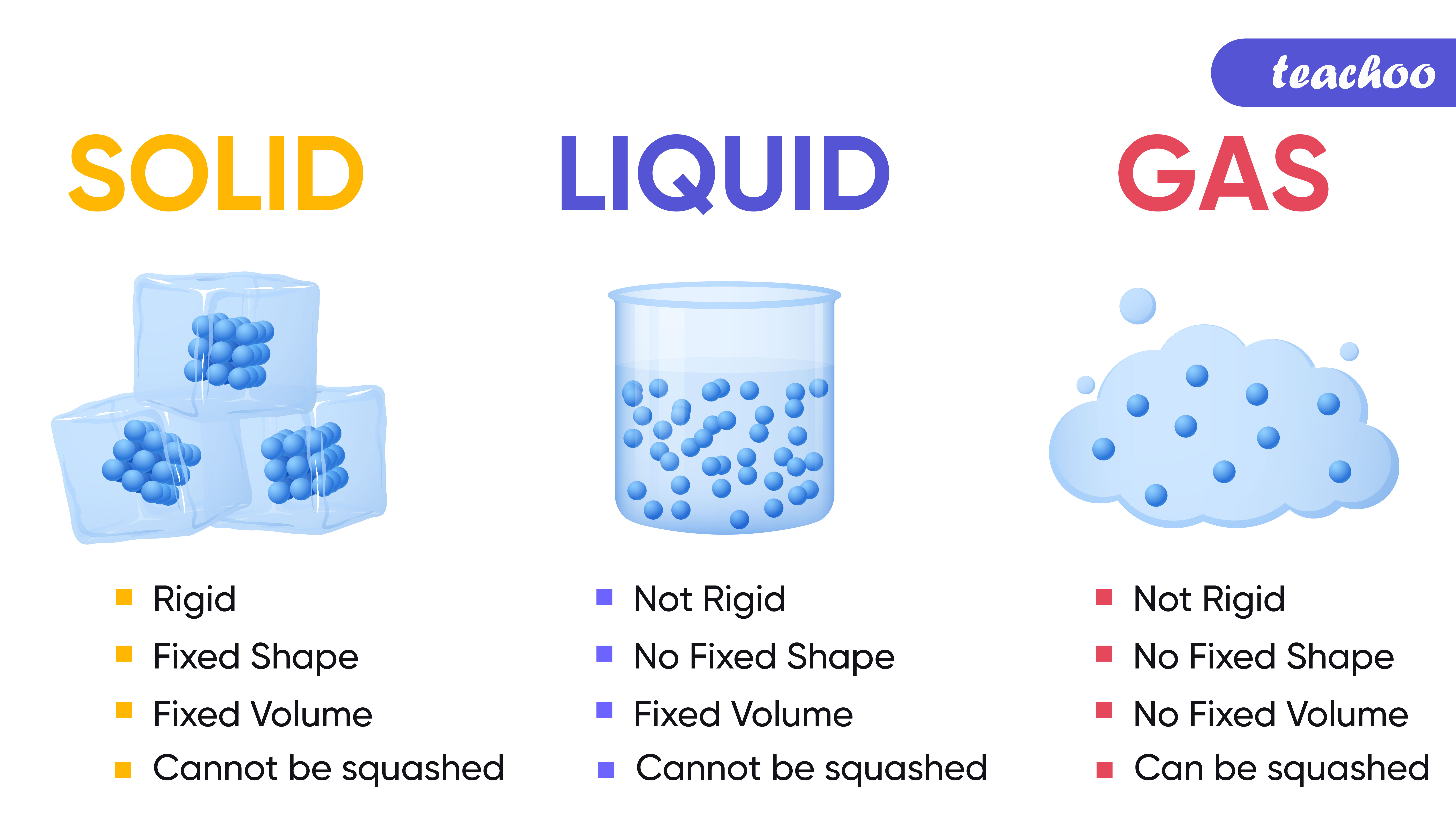
Liquids They can flow or be poured easily. They are not easy to hold. They change their shape depending on the container they are in. Even when liquids change their shape, they always take up.

Q1) solid liquid and gas Science Matter in Our Surroundings
AQA The three states of matter - AQA Solids, liquids and gases The three states of matter can be represented by the particle model. This model explains the properties of substances in their.
PPT APES Unit 2 Abiotic and Biotic Parts of Ecosystems PowerPoint
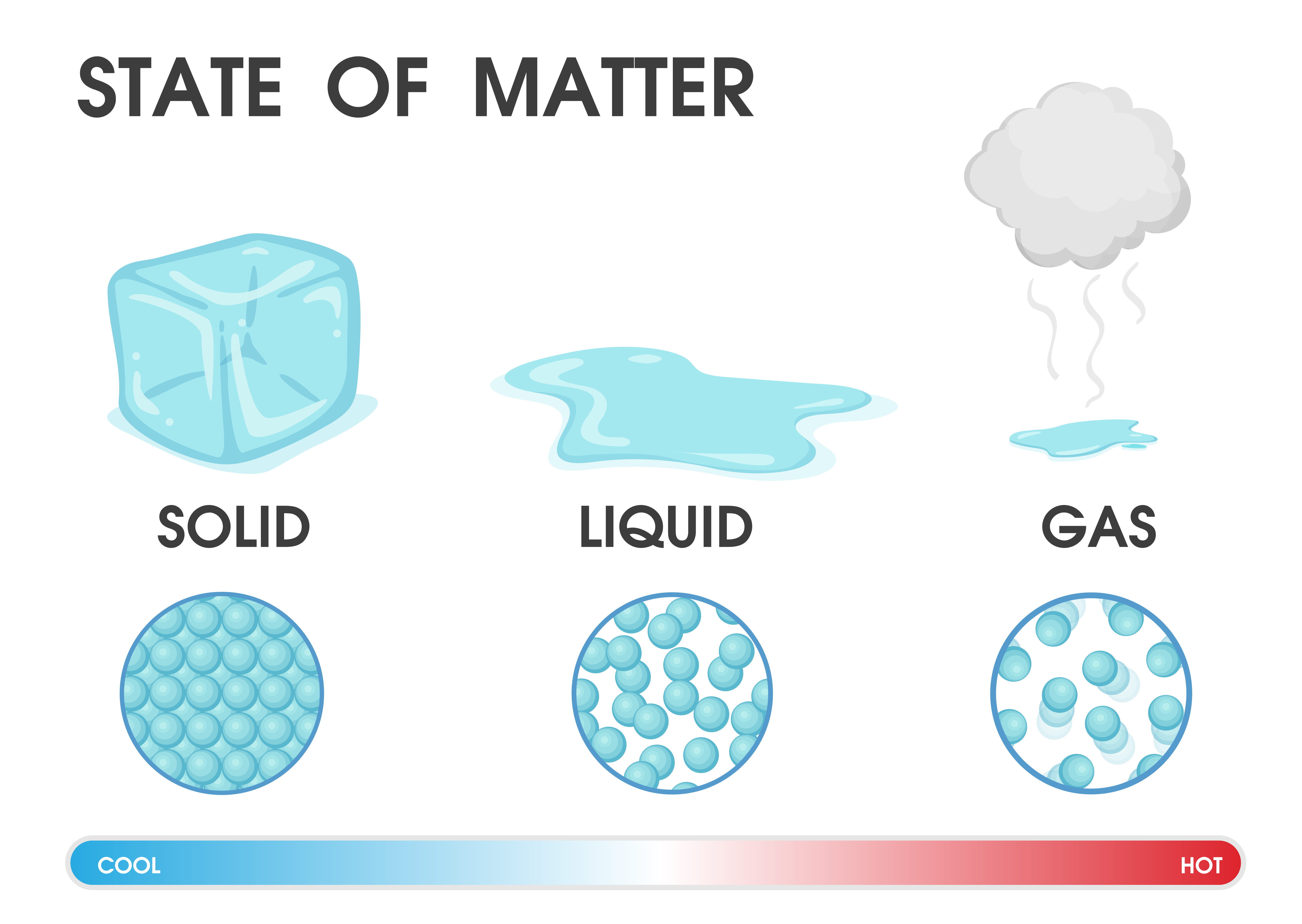
State of matter. Bromine in both liquid and gas state, encased inside acrylic in solid state. In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some.
Q2 Understanding Solids, Liquids and Gases
The solid liquid line is "normal" (meaning positive sloping). For this, complete the following: 1. Roughly sketch the phase diagram, using units of atmosphere and Kelvin. Answer. 1-solid, 2-liquid, 3-gas, 4-supercritical fluid, point O-triple point, C-critical point -78.5 °C (The phase of dry ice changes from solid to gas at -78.5 °C) 2.
Solid Liquid Gas Venn Diagram IAN by Shawn Boggs issuu
Solids, liquids and gases — Science Learning Hub Article Solids, liquids and gases Resource Related topics & concepts Add to collection Water is the only common substance that is naturally found as a solid, liquid or gas. Solids, liquids and gases are known as states of matter.

Solid, Liquid, & Gas Triple Venn Diagram Activity Middle School
AboutTranscript. In this video, we'll learn how to represent solids, liquids, and gases using particulate models. The particles in a solid are either highly ordered (if the solid is crystalline) or have no regular arrangement (if the solid is amorphous). In both cases, the motion of the particles is limited. The particles in a liquid are close.
solid liquid gas drawing
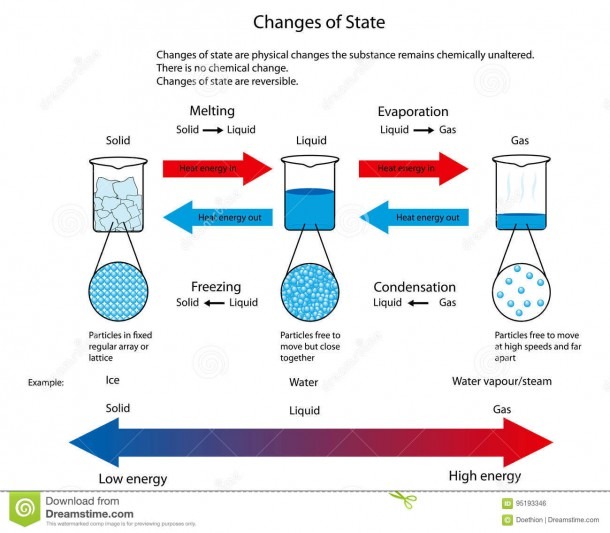
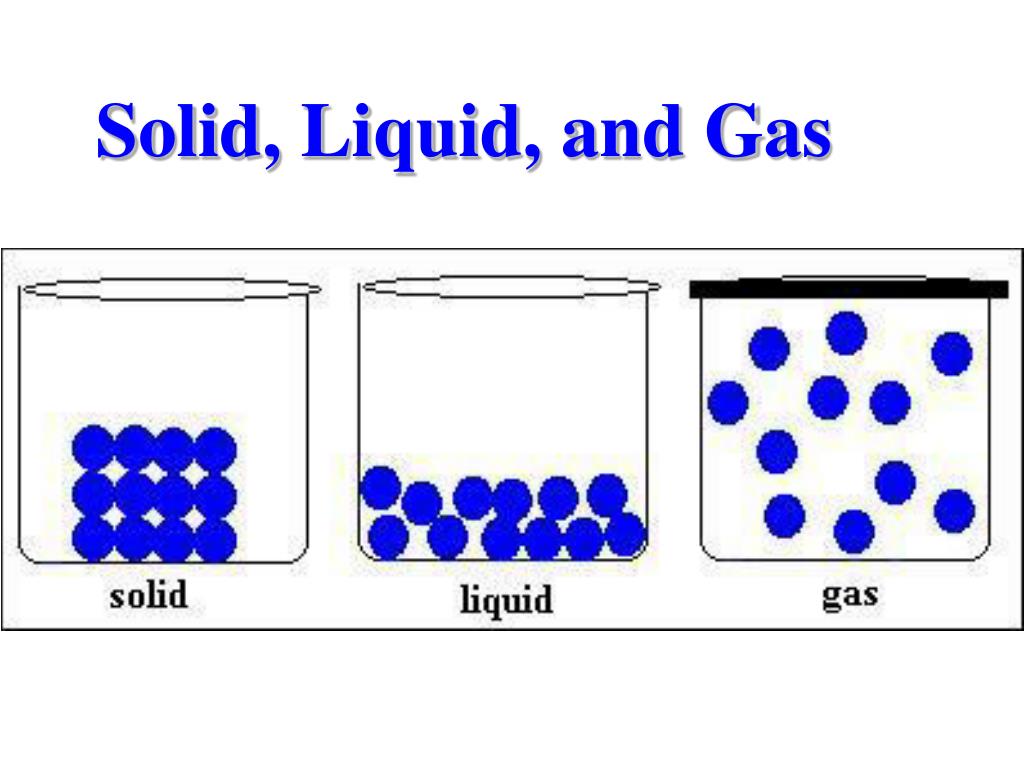
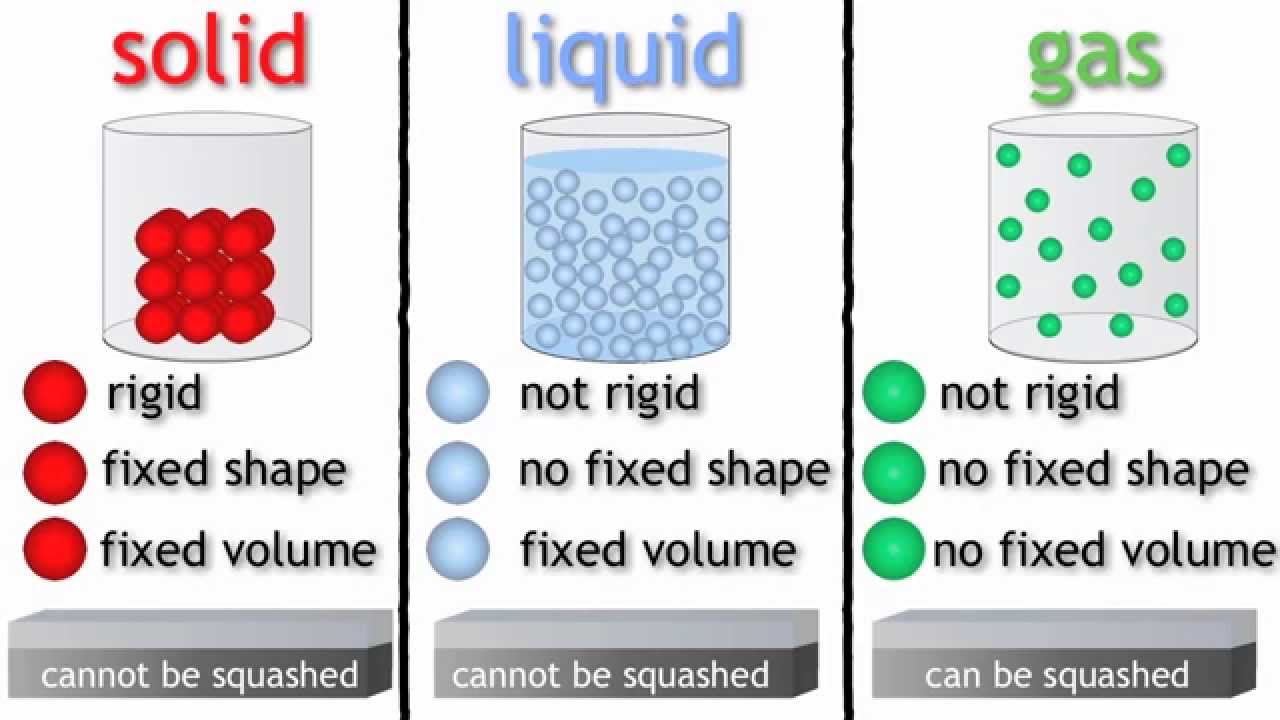
Particles in a: gas vibrate and move freely at high speeds. liquid vibrate, move about, and slide past each other. solid vibrate (jiggle) but generally do not move from place to place. Liquids and solids are often referred to as condensed phases because the particles are very close together.
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas
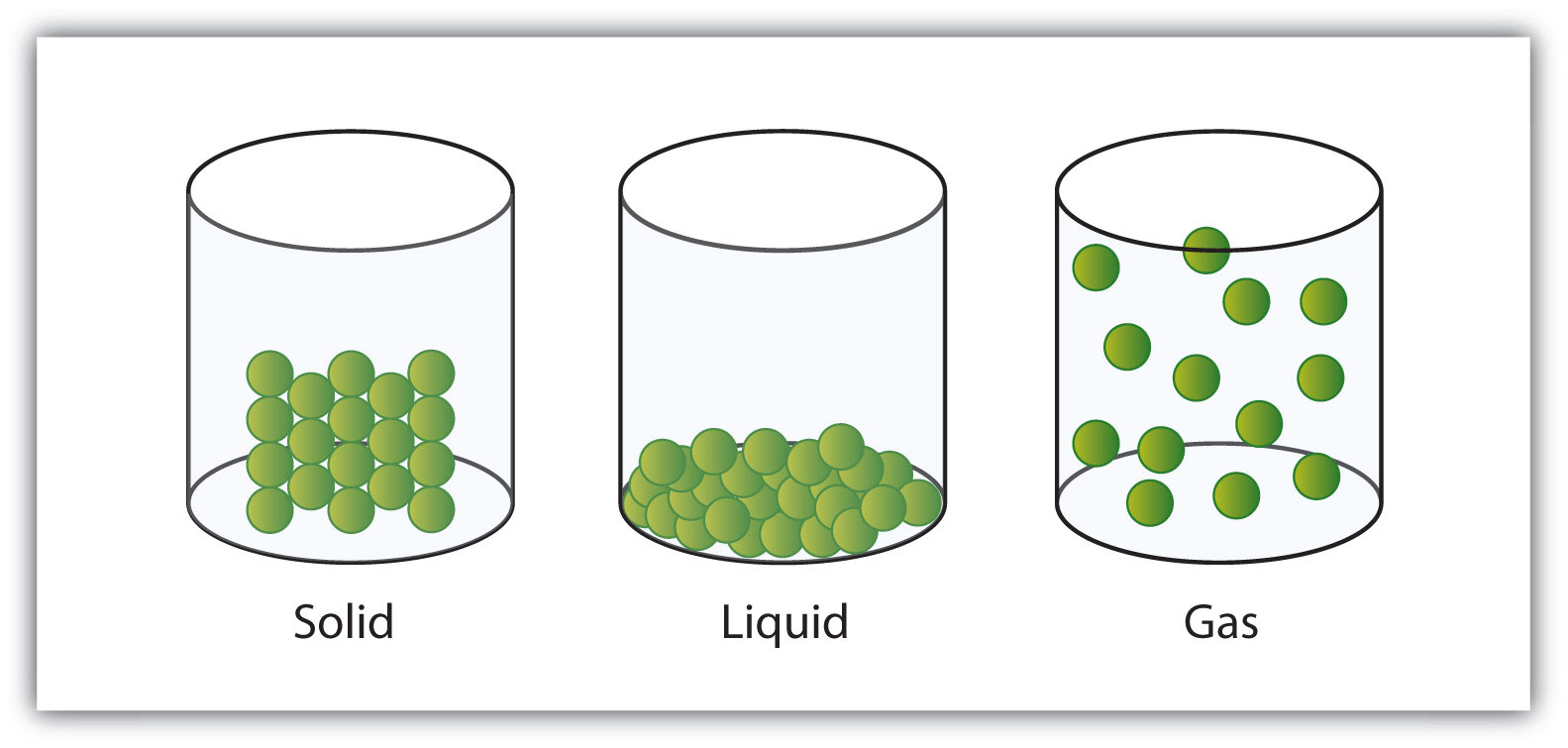
Solids and liquids. The particles of a solid are very close together.It melts when it changes from the solid state to the liquid state. The particles of a liquid remain close together, so there is.

Pin de tintinwin en nilar Estados de agregación de la materia
The intersection with the logarithmic curve for the gas will define an equilibrium pressure for gas-solid co-existence. Generally vapor pressures above solids are quite small, but not negligible. As for liquids we can construct a line representing the equilibrium pressures for sublimation as function of temperature and add it to the phase diagram.
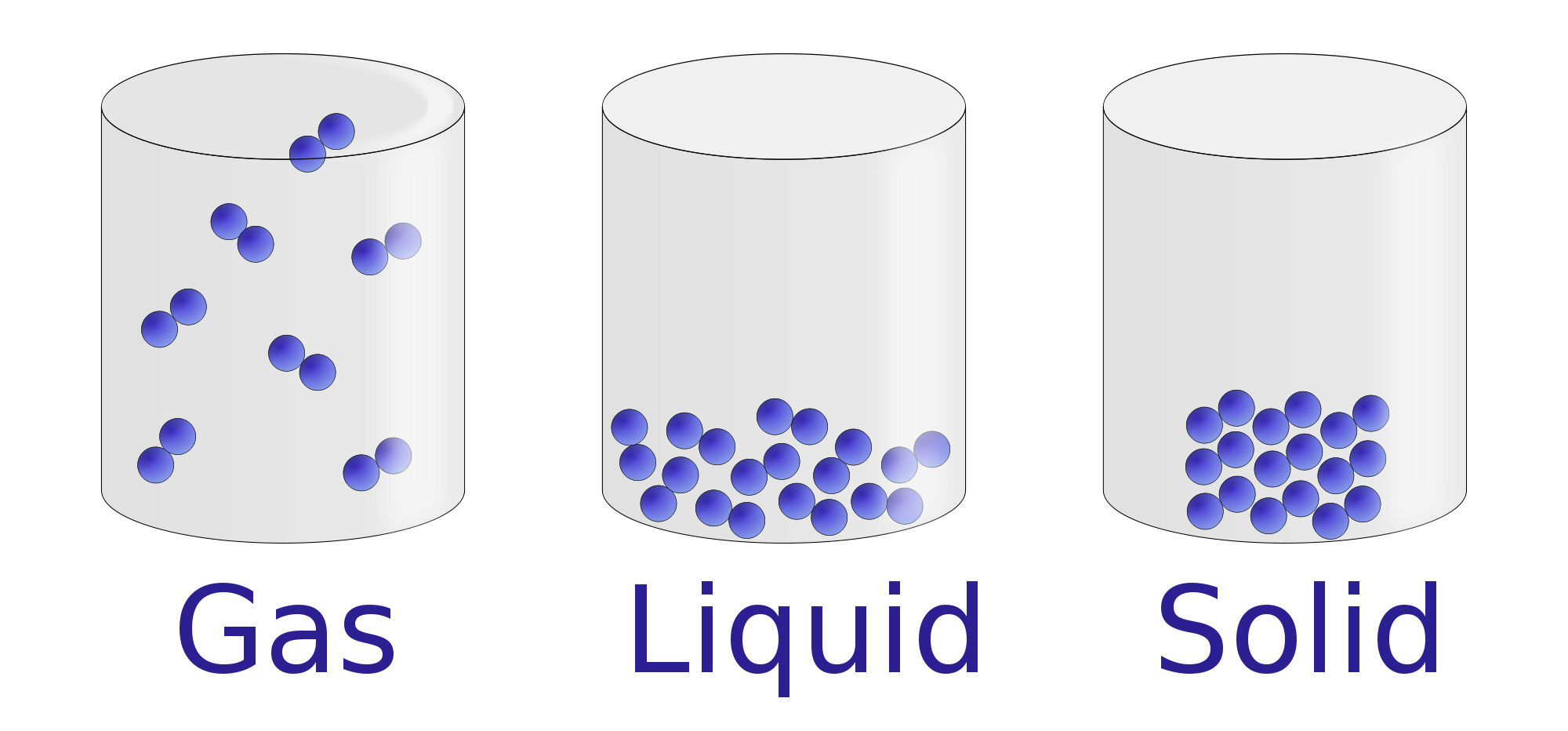
Properties of Liquids Chemistry Visionlearning
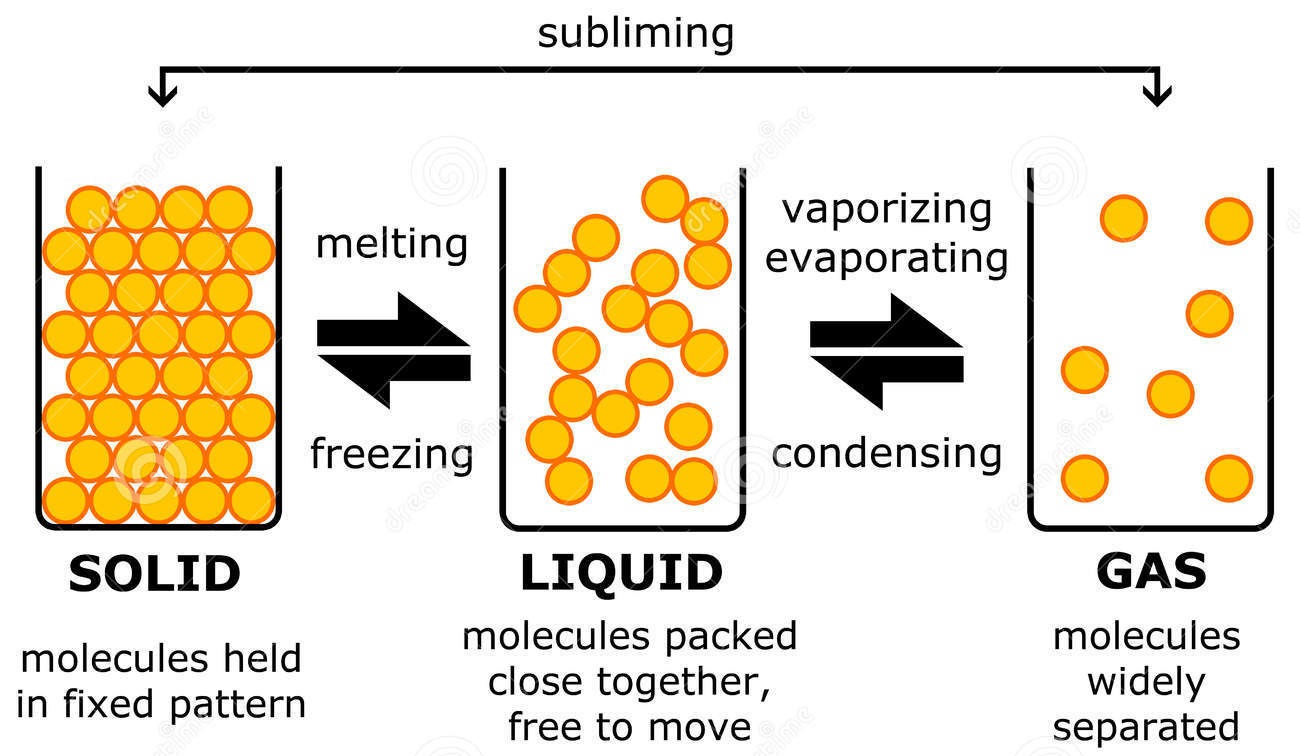
Solids . A solid has a definite shape and volume because the molecules that make up the solid are packed closely together and move slowly. Solids are often crystalline; examples of crystalline solids include table salt, sugar, diamonds, and many other minerals. Solids are sometimes formed when liquids or gases are cooled; ice is an example of a cooled liquid which has become solid.
Physics Matter Online Education System
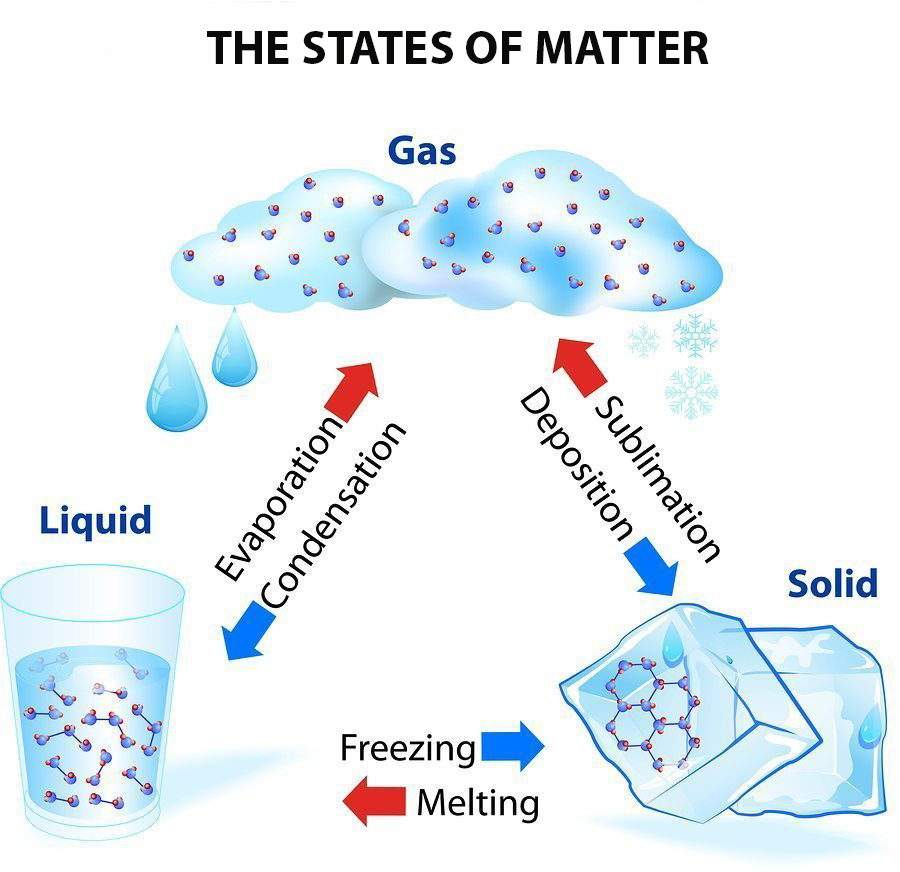
The change from solid to liquid usually does not significantly change the volume of a substance. However, the change from a liquid to a gas significantly increases the volume of a substance, by a factor of 1,000 or more. Figures \(\PageIndex{3}\) and \(\PageIndex{4}\) show the differences among solids, liquids, and gases at the molecular level.

Solids, Liquids, & Gases! Rachel A Tall Drink of Water
Solids: have a fixed volume and a fixed shape cannot flow, because their particles cannot move from place to place cannot be easily compressed , because their particles are close together with no.
Solids, Liquids, and Gases
12: Intermolecular Forces: Liquids And Solids
How does the arrangement of the particles in a liquid compare to that
What are these known as? Changes of state Many substances can exist as solids, liquids or gases, which are all different states of matter. By heating or cooling a substance, its state can be.